Counselors - Peers - Marriage and Family Therapists - Addiction Counselors
Psychologists - Social Workers - Pre-licensure Professionals - Interns
...for a diverse, competent, and sustainable behavioral healthcare workforce.
- Home
- State Resources
- HCPF (Medicaid)
- Medical Loss Ratio (MLR)
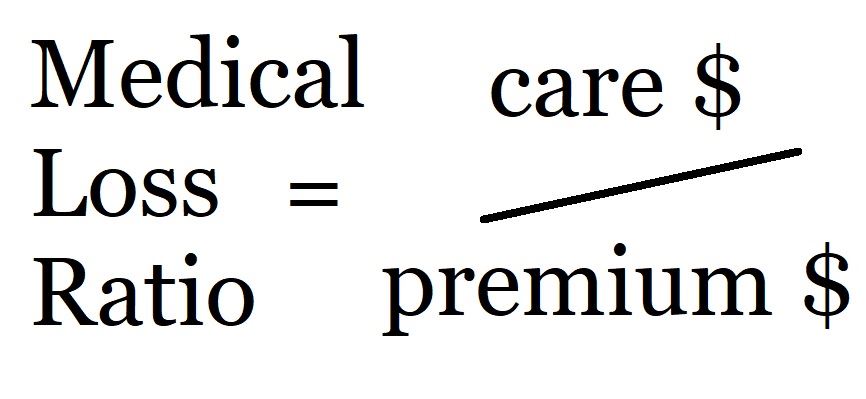
Under managed care, the managed care entity receives payment from the state to provide care. To assure that care is provided, the federal and state governments rely on a measure called the Medical Loss Ratio (MLR). For Medicaid in Colorado, the managed care entities must spend 85% of what they receive on care. Because there is currently (2024) no public data regarding what the RAEs spend, CORA requests and other sources of data such as the APCD (All Payers Claims Database) must stand in the place of an open, transparent reporting process.
It is an ratio, or a fraction, with a numerator and a denominator. Generally the calculation is ($ for care) / ($ received).
HCPF contracts with RAEs typically require, "14.14.1. The Contractor shall calculate and report the MLR according to the instructions provided on the MLR template and the guidance provided in 42 C.F.R. § 438.8(a)."
From that federal rule, here are expenses that may be included in the numerator:
- incurred claims
- expenditures for activities that improve health care quality
- fraud prevention activities
Note that "amounts paid to third party vendors for network development, administrative fees, claims processing, and utilization management," may not be included in the numerator. Many Colorado RAEs contract with third party vendors for these services (RAE 2 Northeast Health Partners for example contracts with Carelon/Beacon/Elevance/Anthem/Amerigroup for claims processing.
Activities that improve health care quality must be in one of the following categories:
- activity that meets the requirements of 45 CFR 158.150(b) and is not excluded under 45 CFR 158.150(c). See below for discussion.
- activity related to any EQR-related activity as described in § 438.358(b) and (c).
- expenditure that is related to Health Information Technology and meaningful use, meets the requirements placed on issuers found in 45 CFR 158.151, and is not considered incurred claims, as defined in paragraph (e)(2) of this section.
Reports
"The State must require each managed care entitiy to submit a report to the State that includes at least the following information for each MLR reporting year:
- (i) Total incurred claims.
- (ii) Expenditures on quality improving activities.
- (iii) Fraud prevention activities as defined in paragraph (e)(4) of this section.
- (iv) Non-claims costs.
- (v) Premium revenue.
- (vi) Taxes, licensing and regulatory fees.
- (vii) Methodology(ies) for allocation of expenditures.
- (viii) Any credibility adjustment applied.
- (ix) The calculated MLR.
- (x) Any remittance owed to the State, if applicable.
- (xi) A comparison of the information reported in this paragraph with the audited financial report required under § 438.3(m).
- (xii) A description of the aggregation method used under paragraph (i) of this section.
- (xiii) The number of member months.
Details about the numerator:
§ 158.150 Activities that improve health care quality.
(1) The activity must be designed to:
- (i) Improve health quality.
- (ii) Increase the likelihood of desired health outcomes in ways that are capable of being objectively measured and of producing verifiable results and achievements.
- (iii) Be directed toward individual enrollees or incurred for the benefit of specified segments of enrollees or provide health improvements to the population beyond those enrolled in coverage as long as no additional costs are incurred due to the non- enrollees.
- (iv) Be grounded in evidence-based medicine, widely accepted best clinical practice, or criteria issued by recognized professional medical associations, accreditation bodies, government agencies or other nationally recognized health care quality organizations.
(2) The activity must be primarily designed to:
(i) Improve health outcomes including increasing the likelihood of desired outcomes compared to a baseline and reduce health disparities among specified populations.
(A) Examples include the direct interaction of the issuer (including those services delegated by contract for which the issuer retains ultimate responsibility under the insurance policy), providers and the enrollee or the enrollee's representative (for example, face-to-face, telephonic, web-based interactions or other means of communication) to improve health outcomes, including activities such as:
- (1) Effective case management, care coordination, chronic disease management, and medication and care compliance initiatives including through the use of the medical homes model as defined in section 3502 of the Affordable Care Act.
- (2) Identifying and addressing ethnic, cultural or racial disparities in effectiveness of identified best clinical practices and evidence based medicine.
- (3) Quality reporting and documentation of care in non-electronic format.
- (4) Health information technology to support these activities.
- (5) Accreditation fees directly related to quality of care activities.
- (6) Commencing with the 2012 reporting year and extending through the first reporting year in which the Secretary requires ICD–10 as the standard medical data code set, implementing ICD–10 code sets that are designed to improve quality and are adopted pursuant to the Health Insurance Portability and Accountability Act (HIPAA), 42 U.S.C. 1320d-2, as amended, limited to 0.3 percent of an issuer's earned premium as defined in § 158.130.
(ii) Prevent hospital readmissions through a comprehensive program for hospital discharge. Examples include:
- (A) Comprehensive discharge planning (for example, arranging and managing transitions from one setting to another, such as hospital discharge to home or to a rehabilitation center) in order to help assure appropriate care that will, in all likelihood, avoid readmission to the hospital;
- (B) Patient-centered education and counseling.
- (C) Personalized post-discharge reinforcement and counseling by an appropriate health care professional.
- (D) Any quality reporting and related documentation in non-electronic form for activities to prevent hospital readmission.
- (E) Health information technology to support these activities.
(iii) Improve patient safety, reduce medical errors, and lower infection and mortality rates.
(A) Examples of activities primarily designed to improve patient safety, reduce medical errors, and lower infection and mortality rates include:
- (1) The appropriate identification and use of best clinical practices to avoid harm.
- (2) Activities to identify and encourage evidence-based medicine in addressing independently identified and documented clinical errors or safety concerns.
- (3) Activities to lower the risk of facility-acquired infections.
- (4) Prospective prescription drug Utilization Review aimed at identifying potential adverse drug interactions.
- (5) Any quality reporting and related documentation in non-electronic form for activities that improve patient safety and reduce medical errors.
- (6) Health information technology to support these activities.
(iv) Implement, promote, and increase wellness and health activities:
(A) Examples of activities primarily designed to implement, promote, and increase wellness and health activities, include—
- (1) Wellness assessments;
- (2) Wellness/lifestyle coaching programs designed to achieve specific and measurable improvements;
- (3) Coaching programs designed to educate individuals on clinically effective methods for dealing with a specific chronic disease or condition;
- (4) Public health education campaigns that are performed in conjunction with State or local health departments;
- (5)(ii) Beginning with the 2021 MLR reporting year, actual rewards, incentives, bonuses, reductions in copayments (excluding administration of such programs) that are not already reflected in premiums or claims, to the extent permitted by section 2705 of the PHS Act;
- (6) Any quality reporting and related documentation in non-electronic form for wellness and health promotion activities;
- (7) Coaching or education programs and health promotion activities designed to change member behavior and conditions (for example, smoking or obesity); and
- (8) Health information technology to support these activities.
(v) Enhance the use of health care data to improve quality, transparency, and outcomes and support meaningful use of health information technology consistent with § 158.151 of this subpart.
(c) Exclusions. Expenditures and activities that must not be included in quality improving activities are:
- (1) Those that are designed primarily to control or contain costs;
- (2) The pro rata share of expenses that are for lines of business or products other than those being reported, including but not limited to, those that are for or benefit self-funded plans;
- (3) Those which otherwise meet the definitions for quality improvement activities but which were paid for with grant money or other funding separate from premium revenue;
- (4) Those activities that can be billed or allocated by a provider for care delivery and which are, therefore, reimbursed as clinical services;
- (5) Establishing or maintaining a claims adjudication system, including costs directly related to upgrades in health information technology that are designed primarily or solely to improve claims payment capabilities or to meet regulatory requirements for processing claims, including maintenance of ICD–10 code sets adopted pursuant to the Health Insurance Portability and Accountability Act (HIPAA), 42 U.S.C. 1320d–2, as amended.
- (6) That portion of the activities of health care professional hotlines that does not meet the definition of activities that improve health quality;
- (7) All retrospective and concurrent utilization review;
- (8) Fraud prevention activities;
- (9) The cost of developing and executing provider contracts and fees associated with establishing or managing a provider network, including fees paid to a vendor for the same reason;
- (10) Provider credentialing;
- (11) Marketing expenses;
- (12) Costs associated with calculating and administering individual enrollee or employee incentives;
- (13) That portion of prospective utilization that does not meet the definition of activities that improve health quality; and
- (14) Any function or activity not expressly included in paragraph (a) or (b) of this section, unless otherwise approved by and within the discretion of the Secretary, upon adequate showing by the issuer that the activity's costs support the definitions and purposes in this part or otherwise support monitoring, measuring or reporting health care quality improvement.
§ 438.358 Activities related to external quality review. (EQR)
(a) General rule.
(1) The State, its agent that is not an MCO, PIHP, PAHP, or PCCM entity (described in § 438.310(c)(2)), or an EQRO may perform the mandatory and optional EQR-related activities in this section.
(2) The data obtained from the mandatory and optional EQR-related activities in this section must be used for the annual EQR in § 438.350 and must include, at a minimum, the elements in § 438.364(a)(2)(i) through (iv).
(b) Mandatory activities.
(1) For each MCO, PIHP, or PAHP the following EQR-related activities must be performed:
- (i) Validation of performance improvement projects required in accordance with § 438.330(b)(1) that were underway during the preceding 12 months.
- (ii) Validation of MCO, PIHP, or PAHP performance measures required in accordance with § 438.330(b)(2) or MCO, PIHP, or PAHP performance measures calculated by the State during the preceding 12 months.
- (iii) A review, conducted within the previous 3-year period, to determine the MCO's, PIHP's, or PAHP's compliance with the standards set forth in subpart D of this part, the disenrollment requirements and limitations described in § 438.56, the enrollee rights requirements described in § 438.100, the emergency and post-stabilization services requirements described in § 438.114, and the quality assessment and performance improvement requirements described in § 438.330.
- (iv) Validation of MCO, PIHP, or PAHP network adequacy during the preceding 12 months to comply with requirements set forth in § 438.68 and, if the State enrolls Indians in the MCO, PIHP, or PAHP, § 438.14(b)(1).
(2) For each PCCM entity (described in § 438.310(c)(2)), the EQR-related activities in paragraphs (b)(1)(ii) and (iii) of this section must be performed.
(c) Optional activities. For each MCO, PIHP, PAHP, and PCCM entity (described in § 438.310(c)(2)), the following activities may be performed by using information derived during the preceding 12 months:
- (1) Validation of encounter data reported by an MCO, PIHP, PAHP, or PCCM entity (described in § 438.310(c)(2)).
- (2) Administration or validation of consumer or provider surveys of quality of care.
- (3) Calculation of performance measures in addition to those reported by an MCO, PIHP, PAHP, or PCCM entity (described in § 438.310(c)(2)) and validated by an EQRO in accordance with paragraph (b)(1)(ii) of this section.
- (4) Conduct of performance improvement projects in addition to those conducted by an MCO, PIHP, PAHP, or PCCM entity (described in § 438.310(c)(2)) and validated by an EQRO in accordance with paragraph (b)(1)(i) of this section.
- (5) Conduct of studies on quality that focus on a particular aspect of clinical or nonclinical services at a point in time.
- (6) Assist with the quality rating of MCOs, PIHPs, and PAHPs consistent with § 438.334.
(d) Technical assistance. The EQRO may, at the State's direction, provide technical guidance to groups of MCOs, PIHPs, PAHPs, or PCCM entities (described in § 438.310(c)(2)) to assist them in conducting activities related to the mandatory and optional activities described in this section that provide information for the EQR and the resulting EQR technical report.
[81 FR 27853, May 6, 2016, as amended at 82 FR 39, Jan. 3, 2017; 82 FR 12510, Mar. 6, 2017; 85 FR 72841, Nov. 13, 2020]
158.151 Expenditures related to Health Information Technology and meaningful use requirements.
(a) General requirements. An issuer may include as activities that improve health care quality such Health Information Technology (HIT) expenses as are required to accomplish the activities allowed in § 158.150 of this subpart and that are designed for use by health plans, health care providers, or enrollees for the electronic creation, maintenance, access, or exchange of health information, as well as those consistent with Medicare and/or Medicaid meaningful use requirements, and which may in whole or in part improve quality of care, or provide the technological infrastructure to enhance current quality improvement or make new quality improvement initiatives possible by doing one or more of the following:
- (1) Making incentive payments to health care providers for the adoption of certified electronic health record technologies and their “meaningful use” as defined by HHS to the extent such payments are not included in reimbursement for clinical services as defined in § 158.140 of this subpart;
- (2) Implementing systems to track and verify the adoption and meaningful use of certified electronic health records technologies by health care providers, including those not eligible for Medicare and Medicaid incentive payments;
- (3) Providing technical assistance to support adoption and meaningful use of certified electronic health records technologies;
- (4) Monitoring, measuring, or reporting clinical effectiveness including reporting and analysis of costs related to maintaining accreditation by nationally recognized accrediting organizations such as NCQA or URAC, or costs for public reporting of quality of care, including costs specifically required to make accurate determinations of defined measures (for example, CAHPS surveys or chart review of HEDIS measures and costs for public reporting mandated or encouraged by law.
- (5) Tracking whether a specific class of medical interventions or a bundle of related services leads to better patient outcomes.
- (6) Advancing the ability of enrollees, providers, issuers or other systems to communicate patient centered clinical or medical information rapidly, accurately and efficiently to determine patient status, avoid harmful drug interactions or direct appropriate care, which may include electronic Health Records accessible by enrollees and appropriate providers to monitor and document an individual patient's medical history and to support care management.
- (7) Reformatting, transmitting or reporting data to national or international government-based health organizations for the purposes of identifying or treating specific conditions or controlling the spread of disease.
- (8) Provision of electronic health records, patient portals, and tools to facilitate patient self-management.
